People want to smooth smile lines, crow’s feet, and plump their lips, cheeks, and hands. Dermal fillers can reduce facial wrinkles and volume loss caused by age or medical diseases in the face and hands. People frequently report satisfaction with FDA-approved dermal fillers.
Dermal fillers aren’t for everyone. Bleeding problems and some allergies may preclude dermal fillers. All medical products have pros and cons if your doctor recommends dermal fillers.
The FDA recommends working with a certified healthcare provider experienced in injecting dermal fillers who knows about fillers, anatomy, and problems. Most importantly, it notifies you about the risks and benefits before treatment.
Our med spa only works with experienced healthcare providers for safe dermal filler injections, and we prioritize educating clients on risks and benefits beforehand.
How Do Dermal Fillers Work?
Dermal fillers are substances injected under the skin and have a gel-like substance. The purpose of dermal fillers is to provide an appearance that is either smoother, fuller, or both. Being medical devices, dermal fillers are subject to regulation by the FDA. Most FDA-approved Dermal fillers have only temporary benefits since they are constructed from components the body will eventually break down and absorb. This was discovered through clinical testing. The injection process may need to be repeated to keep the intended result.
What Are The Types Of Dermal Fillers?
The following types of materials are considered to be temporary fillers:
- Hyaluronic acid is a type of sugar found in the human body naturally.
- Calcium hydroxylapatite is a mineral that makes up a significant portion of bone.
- Poly-L-lactic acid, sometimes known as PLLA, is a synthetic biodegradable substance.
There is only FDA-approved dermal filler that is not absorbed by the body. It is made with bovine (cow) collagen-containing beads made of polymethylmethacrylate (PMMA) suspended in a solution. Beads made of polymethyl methacrylate (PMMA) are tiny, spherical, and smooth.
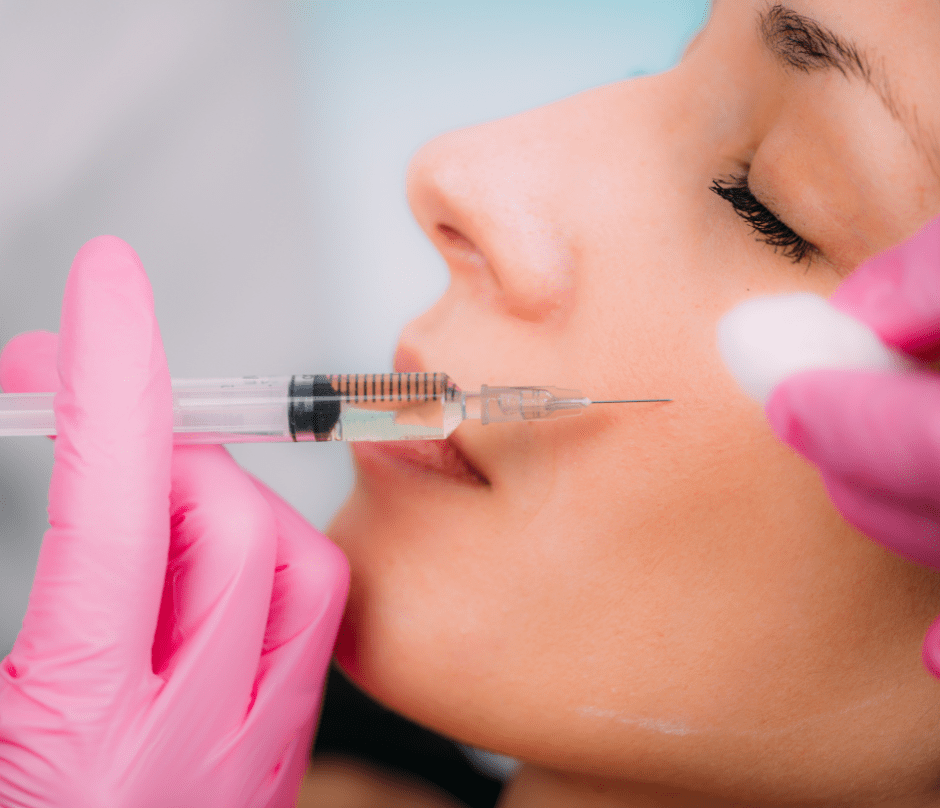
The FDA-Approved Dermal Fillers Uses
Dermal fillers can be used in specific ways by adults 22 and older. They consist of the following:
- Increased fullness in the lips, cheeks, chin, under-eye hollows, jawline, and back of the hand.
- Removing skin folds and moderate-to-severe face wrinkles.
- Getting rid of scars left by acne on the cheeks
- Reversing the loss of facial fat in HIV-positive individuals (HIV).
The FDA Cautions Against Unapproved Fillers
- Hyaluronic acid and other lip and facial fillers should not be injected using needle-free devices because the FDA has not approved injecting dermal fillers. The injectors’ high pressure does not give the user enough control over where the filler will be applied. There have been severe wounds and, in some cases, irreversible damage to the skin, lips, or eyes.
- The FDA also advises against using direct-to-consumer lip and face fillers. They’re not FDA-approved and may include chemicals and pathogens. Only licensed healthcare professionals can inject FDA-approved dermal fillers using a syringe, needle, or cannula.
- Injectable silicone or fillers for body contouring or enlargement are not FDA-approved. The FDA advises against breast, buttock, and muscular filler injections. Large-scale body contouring or enlargement with injectable filler can cause long-term pain, infection, scarring, and death.
We will talk more about the FDA-approved Dermal fillers in [Part 2], so be ready.


